Fluticasone nasal spray recall
Dr. Steven encourages patients to check their fluticasone nasal spray bottle to see if it was made by Apotex; they are recalling one lot because of contamination with small glass particles. The only reported problem is that some of the bottles have become plugged by the particles. Nobody has been reported to have been injured from spraying glass particles into their nose, but that is possible. Thus, if you have a bottle of fluticasone nasal spray made by Apotex, read more below and follow the link to the FDA?s website that explains how to tell if your bottle is being recalled, and what you can do to get it replaced.

From the FDA:
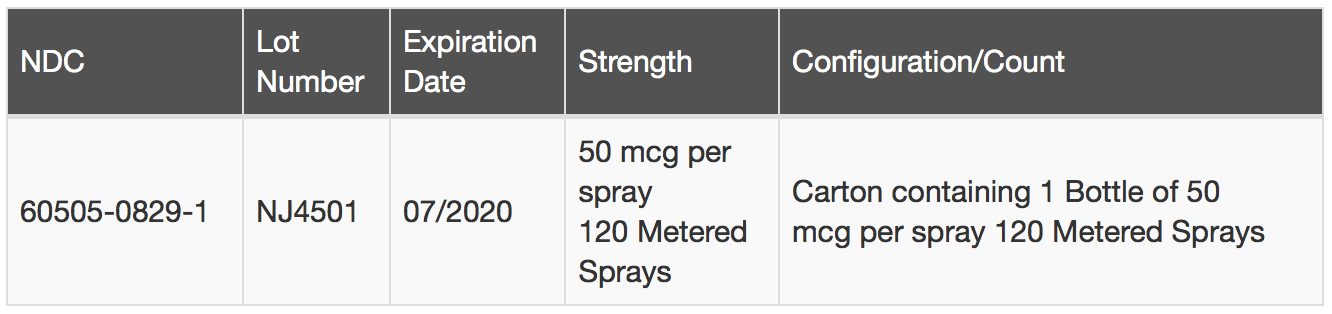
Apotex Corp. is voluntarily recalling one (1) lot of Fluticasone Propionate Nasal Spray, USP, 50 mcg per spray, 120 Metered Sprays, to the consumer level. The Fluticasone Propionate Nasal Spray USP 50 mcg per spray 120 Metered Sprays has been found to contain small glass particles. The glass particles could block the actuator and impact the functionality of the pump. The issue was discovered through a customer complaint.
Risk Statement: There is a potential for patients to be exposed to the glass particles and mechanical irritation cannot be ruled out. Local trauma to the nasal mucosa might occur with use of the defective product. To date, Apotex Corp. has not received any reports of adverse events related to recall.
Fluticasone Propionate Nasal Spray USP 50 mcg per spray 120 Metered Sprays is indicated for the treatment of seasonal and perennial allergic rhinitis and for the management of sinus pain and pressure associated with allergic rhinitis in patients 4 to 17 years of age The affected Fluticasone Propionate Nasal Spray USP 50 mcg per spray 120 Metered Sprays can be identified by the information in the table below: and on the product label: